Abstract
Development of a Redox-Active Ligand Scaffold for the Activation of Lactide
 Students: Sarah Bergman, James Zoller, and Lauren Boedicker
Students: Sarah Bergman, James Zoller, and Lauren Boedicker
Mentor: Allen Pistner (OWU Department of Chemistry)
We are developing a catalyst for producing an environmentally-friendly plastic called polylactide, which is used for making biodegradable packaging material and utensils. This catalyst will hopefully be cheaper than those currently used, and allow for greater control over the properties of the polymer. We are currently attempting two different synthetic pathways to try to find the more efficient route.
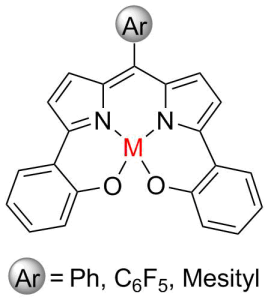
A redox active ligand scaffold for the ring-opening polymerization of lactide was synthesized. The construction of the dipyrrin scaffold was approached with two separate synthetic pathways. The first approach begins with the Negishi-type cross coupling of an aryl halide with zincated pyrrole species, followed by the condensation with an aryl aldehyde to afford the dipyrrin structure. The second pathway uses a top-down approach, which begins with the condensation of pyrrole with an aryl aldehyde to form the dipyrromethane, followed by bromination at the α-positions. A Suzuki cross coupling reaction with an aryl boronic acid produces the dipyrrin ligand. Both approaches provide an opportunity to influence the electronic properties of the scaffold through the inclusion of electron-withdrawing or electron-donating aryl groups. Metal complexes will be synthesized and be fully characterized including catalytic activity for the ring-opening polymerization of lactide.